Pharma / MedTech
Cloud-based, real world evidence data management for sites and patients
Connecting the dots
between patients and CROs
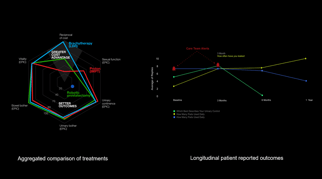
Visiontree is 21 CFR Part 11 compliant cloud-based application for multi-site clinical trial management and patient access to outcomes assessments to deliver real-world evidence research and value-based care reporting solutions.
Visiontree provides library of over 400 electronic, standards-based patient and clinician reported outcomes assessments (ePRO and eCOA), including PROMIS® CAT, CTCAE and PRO-CTCAE, as well as custom form building capabilities. For over a decade, Visiontree has delivered proven, reliable, patient-centered and scalable outcomes solution for pharmaceutical, medtech and biotech clinical trials with industry leading contract research organizations (CROs) and academic hospitals.
Visiontree provides user-friendly clinical trials study management features for patient accrual, eligibility and retention. This includes seamless workflow interoperability with EHRs and research data systems, such as Medidata Rave®, utilizing HL7 FHIR®, APIs and patient access via their own desktop and mobile device.
Outcomes Packages by Specialty
Visiontree provides standards-based ePRO, eCOA and customized solutions in these, and other, core domains.
- Oncology
- Cardiology &
Heart Surgery - Neurological &
Spine Surgery - Orthopaedic
Surgery - Behavioral
Health - Research &
Registries
Interested?
For more information.